How Fast Does Parylene Deposit?
Posted by Sean Horn
Friday, November 20, 2020 8:00
@ 8:00 AM
Parylene conformal coatings combine a number of properties that are attractive for use in a wide spectrum of applications. Their low dielectric properties, high mechanical strength, transparency, bio compatibility, chemical inertness against all of the common acids, bases and organic solvents, low water/gas permeability and thermal properties make them interesting for use in many industries. Also, pinhole-free Parylene conformal coatings with a thickness higher than 0.1 μm are possible and have been reported earlier [1]. Therefore, understanding their deposition process and characteristics is important.
Parylene conformal coatings are deposited using the Gorham chemical vapor deposition (CVD) process under vacuum. (CVD) process of parylene takes place in three steps (sublimation, pyrolysis, deposition) as shown in the Figure. First the dimer (precursor with two repeating units) is sublimed. Next step the dimer is cracked into two monomers by pyrolysis at temperatures above > 500°C. Finally, the monomers polymerize on the substrate surface (as well as chambers surfaces) forming linear polymer chains. This final step takes place at room temperature and stress free conformal coatings are obtained.
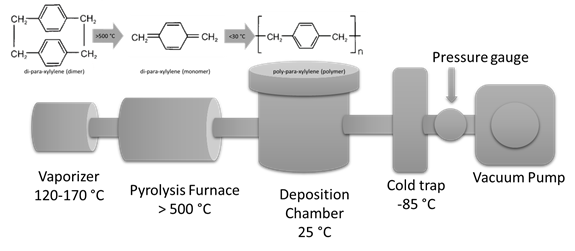
Polymerization route for poly-para-xylylene (Parylene N – C16H16)
Factors affecting the parylene deposition rate and thickness:
The deposition rate and the pressure of Parylene conformal coatings during coating process controls uniformity and surface roughness of the final thin film. High pressure and high deposition rate have been reported to yield rough and non-uniform thin film coatings with poor dielectric properties [1]. Jui-Mei Hsu et al reported that high precursor sublimation rates resulted in slightly higher root-mean-square surface roughnesses from 5.78 to 9.53 nm for deposition rates from 0.015 to 0.08 g/min [2]. Therefore, extra care must be taken when choosing the deposition rate which in turn affects how fast the Parylene can be deposited.
According to Franz Selbmann et al, Eq. (1) and (2) shows that the Parylene thickness s and the deposition duration t increase with increasing dimer mass (amount of dimer used) by a factor of about 0.45 µm/g and 10.80 min/g, respectively. They worked with dimer masses between 1.1 g to 10 g to find a correlation. All depositions were performed at
130°C sublimation temperature and autogenous pressure <5.5 Pa.
s = 0.3386 µm + 0.4528 µm/g · mD (Eq 1)
t = 1.55 min + 10.80 min/g · mD (Eq 2)
They also worked with different deposition parameters to understand their influence on the Parylene coatings. They varied sublimation temperature from 100°C to 200°C with temperature steps of 10°C. They reported the deposition rate as to vary between 20 nm/min for 100°C and 156 nm/min for 200°C. They also showed that the layer thickness was slightly lower for higher sublimation temperatures which was explained by the lower dwell time and the lower reaction time of the monomer molecules[2].
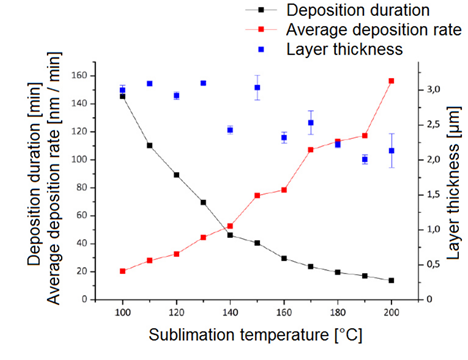
Deposition duration, average deposition rate and layer thickness for different sublimation temperatures between 100-200 °C and constant dimer mass (6 g) for Parylene C. Image Ref: [2]
While parylene coatings are typically thin, they also deposit relatively slowly. The fastest-depositing variant of parylene — Parylene C — typically deposits at a rate of 0.2 mils or 5 microns per hour. This means that a 75 micron coating would take approximately 15 hours. Parylene N and D deposit more slowly.
Overall, the process might take from a few hours to over 24 hours depending on the coating thickness we are aiming for. The time required to reach this thickness is controlled by the dimer mass, deposition rate, temperature and other factors.
To learn more about parylene, download our whitepaper now
References
[1] T. Marszalek, M. Gazicki-Lipman, and J. Ulanski, “Parylene C as a versatile dielectric material for organic field-effect transistors,” Beilstein J. Nanotechnol., vol. 8, no. 1, pp. 1532–1545, Jul. 2017, doi: 10.3762/bjnano.8.155.
[2] F. Selbmann, M. Baum, M. Wiemer, and T. Gessner, “Deposition of Parylene C and characterization of its hermeticity for the encapsulation of MEMS and medical devices,” in 2016 IEEE 11th Annual International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Sendai, Japan, Apr. 2016, pp. 427–432, doi: 10.1109/NEMS.2016.7758283.
Comments
Homepage 4/17/2020. 10:17:10 AM
... [Trackback] [...] Informations on that Topic: blog.paryleneconformalcoating.com/whats-the-difference-between-potting-and-conformal-coating/ [...]

londondrugscanada.bigcartel.comlondon-drugs 4/17/2020. 10:17:10 AM
cialis uk https://londondrugscanada.bigcartel.com/london-drugs This is nicely expressed. !