Parylene and Hydrofluoric Acid
Posted by Sean Horn
Friday, September 18, 2020 8:00
@ 8:00 AM
Parylene is a chemically inert conformal coating [1]. It has a well-established chemical vapor deposition process and patterning methods. It is a great candidate for use in various application areas (health, aerospace, oil and gas, microelectronics, and so on.) due to its mechanical, physical, optical and chemical properties. Parylene is known to withstand highly corrosive environments and it can be utilized as a barrier material against various etchants in different processes (e.g. Hydrofluoric acid (HF), nitric acid, and acetic acid; potassium hydroxide; and tetramethylammonium hydroxide).
Corrosive etchants hinder the production that makes use of by wet chemical methods and prevents use of parts under circumstances where the material must be masked and protected from corrosive liquids and/or gases. Among them Hydrofluoric acid has a wide range of use in the processing of MEMS devices and medical implants.
Spin/spray coatable or brushable polymers such as epoxies, urethanes have been proposed and used against Hydrofluoric acid protection in the industry, however parylene can be conformally coated on all kind of geometric shapes with precisely controlled thicknesses and provides a superior protection. Therefore, it is an attractive protective conformal coating layer compared to all the other proposed methods.
There is no known or reported chemical reaction between parylene and common etchants such as hydrofluoric acid, and one can assume that parylene will not be damaged during etching processes. Several experiments have confirmed this claim [2].
Most research focuses on answering the following questions:
- How thick parylene should be to provide decent protection and
- What is the etching selectivity with respect to the targeted material to be etched in Hydrofluoric acid?
For example, corrosion of surface oxide of polysilicon wafers takes place in the hydrofluoric acid at any concentration, which leads to the change in the surface morphology of the wafer and device failure due to the detachment of electrodes from the electronic devices. Also, Buffered Hydrofluoric acid (BHF) is used to remove surface oxide of electrodes to improve the contacts (Aluminum oxide, Copper oxide, Titanium oxide, etc.) to enable its use a good protection for the rest of the product is essential. Parylene protective coating can prevent the corrosion of masked parts in such kind of cases.
Thickness of the Parylene plays an important role because it is also prone to swelling at lower thicknesses. In a study on the parylene protection of surface micromachined polysilicon film, it was shown that a parylene conformal coating of 0.5–2 μm can withstand 40 min exposure to 40% HF (Fig 1) [2]. In the study, Parylene type C was CVD coated and patterned using an O2 plasma system, A174 adhesion promoter was applied before the deposition process. 40% HF is a very high concentration level and 40 minutes exposure is a relatively long time for such processes. As a conclusion, the thicker parylene is, the better etch-masking ability it provides. Some studies suggest a high temperature annealing, 350°C, for several hours, under vacuum or in an inert environment of Parylene conformal coating to improve its stability in hydrofluoric acid as well [1].
Figure 1 SEM of Al with 2 μm parylene after 40 min HF etching.
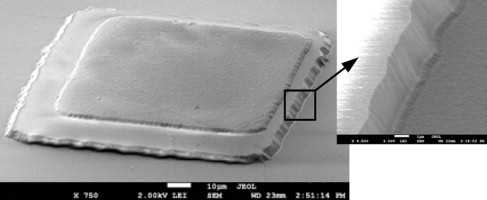
A different group of researchers has shown that Parylene is a decent masking material for use in HF Vapor release for MEMS/NEMS systems [3]. A thick layer of Parylene is sufficient for use in liquid HF or gaseous HF. Lower thickness Parylene/Al/Parylene stack is suggested as a protective coating as well [3].
In conclusion, Parylene is visibly not reacting with hydrofluoric acid and is safe to be used as a protective masking layer (encapsulation) when applicable.
To learn more about parylene, download our whitepaper now:
Common Parylene Problems
References
[1] Hsi-wen Lo, Wen-Cheng Kuo, Yao-Joe Yang, and Yu-Chong Tai, “Recrystallized parylene as a mask for silicon chemical etching,” in 2008 3rd IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Sanya, China, 2008, pp. 881–884, doi: 10.1109/NEMS.2008.4484464.
[2] Y. Zhang, Y. Wang, M. Cai, Y. Wang, Y. Hao, and J. Chen, “Metallization introduced corrosion and parylene protection of surface micromachined polysilicon film with submicron capacitive gap,” Microelectron. Eng., vol. 97, pp. 20–25, Sep. 2012, doi: 10.1016/j.mee.2012.03.010.
[3] A. Higo, K. Takahashi, H. Fujita, Y. Nakano, and H. Toshiyoshi, “A novel Parylene/Al/Parylene sandwich protection mask for HF Vapor release for micro electro mechanical systems,” in TRANSDUCERS 2009 – 2009 International Solid-State Sensors, Actuators and Microsystems Conference, Jun. 2009, pp. 196–199, doi: 10.1109/SENSOR.2009.5285531.
Comments
Homepage 4/17/2020. 10:17:10 AM
... [Trackback] [...] Informations on that Topic: blog.paryleneconformalcoating.com/whats-the-difference-between-potting-and-conformal-coating/ [...]

londondrugscanada.bigcartel.comlondon-drugs 4/17/2020. 10:17:10 AM
cialis uk https://londondrugscanada.bigcartel.com/london-drugs This is nicely expressed. !