What is the Service Life of Parylene?
Posted by Sean Horn
Friday, September 25, 2020 8:00
@ 8:00 AM
Under ambient conditions (room temperature, in air) Parylene is a lifetime conformal coating. The exposure to environmental stressors like temperature, oxygen, UV-light and chemicals can degrade the lifetime especially when two or more are existent at the same time.
Grattan and Bilz, reported that the useful lifetime of Parylene as a conformal coating at 25°C (room temperature) (without light exposure) is 2,200 years (Parylene N) and 130,000 years (Parylene C)[1]. Thermal endurance of Parylene N, C and D are summarized in Figure1[2].
Tensile strength and Thermal Endurance: Free standing parylene films have been reported to be used during tensile strength tests and a 50% loss in the tensile strength have been used as the failure criterion in the study. In parylene samples, the tensile strength was maintained until the entanglement is no longer a factor and after that point the tensile strength drops abruptly [2].
According to Figure 1 Parylene D has the longest stability compared to C and C has a better lifetime compared N. It must be kept in mind that, once these films are exposed to light the useful lifetime decreases further due to oxidation and the molecular chains absorb oxygen and form carbonyl chains. Researchers suggest that quantification of oxidation products is possible and the consumption of oxygen leads to an increase in the spectrum fingerprint of carbonyl peak (1700cm-1 for Parylene N and 1695cm-1 for Parylene C). The rate of oxidation decreases in time probably due to the decrease in the amount of available polymer chains available for oxidation. The degradation becomes inevitable under these conditions. The structure can not serve as a protective layer anymore. As films get oxidized yellowing and embrittlement was observed by researchers and they reported that the onset of yellowing occurred in the following order: Parylene N, Parylene C [1].
Parylene F can withstand oxidation better than the latter under UV exposure and higher temperatures. Finally, Parylene AF-4’s melting point is greater than 500° C. It survives at higher temperatures/UV-exposure better than other parylenes for long durations because it possesses CF2 units, situated between its polymer-chain rings.
|
|
25 °C |
60 °C |
100 °C |
150 °C |
|
Parylene AF4 |
Longer |
Longer |
Longer |
Longer |
|
Parylene F |
Longer |
Longer |
Longer |
Longer |
|
Parylene D |
Longer |
Longer |
10 years |
˂1 day |
|
Parylene C |
130,000 years |
15 years |
˂1 year |
˂˂1 day |
|
Parylene C UV exposure |
2.34 years |
|
|
|
|
Parylene N |
3880 years |
10 years |
˂1 month |
˂˂˂1 day |
|
Parylene N UV exposure |
0.31 years |
|
|
|
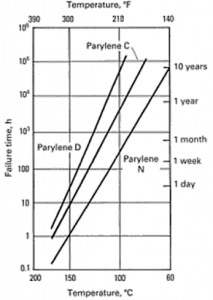
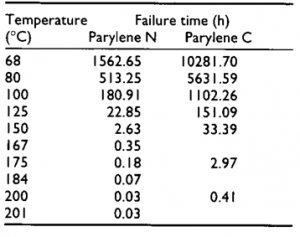
Figure 1 Useful lifetime of Parylene N, C and D as a function of temperature in air. Failure: 50% loss in tensile strength [2]. Left (Table showing the lifetime of Parylene N and D) [3].
The effect of UV exposure and Lifetime:
Grattan and Bilz later carried out another study on Parylene C and N to find out about the effect of UV light exposure on the oxidation. They set 0.061 M carbonyl concentration as the criterion for film failure and find out that under UV exposure of 2500 lux Parylene N can withstand 2784 hours (0.31 years) and Parylene C can withstand 20,538 hours (2.34 years) at room temperature (As shown in Figure 2) [3].
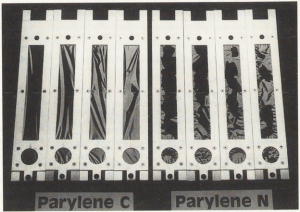
Figure 2 After 3 years of exposure to 2500 lux of UV exposure. Parylene C intact and Parylene N broken.
The huge difference between the failure time between UV exposed films and films that are not exposed gives an important insight about the proper service conditions of Parylene C and N. The chemical structure of Parylene D, C, N, AF4 defines the degradation due to UV exposure. Parylene N is an unsubstituted hydrocarbon molecule, Parylene C has one added chlorine group per repeat unit, Parylene D has two added chlorine groups per repeat unit. In contrast, Parylene AF4 replaces the hydrogen atoms on the chemical’s benzene ring with fluorine atoms, significantly enhancing its UV-stability. To read more about Parylene AF4 you can visit the our blog.
It is important to note that the mechanical properties of Parylene can be improved using thermal treatment. This also improves the lifetime [4].
To learn more about parylene, download our whitepaper now:
Parylene & UV Light Whitepaper
References
[1] D. W. Grattan and M. Bilz, “The Thermal Aging of Parylene and the Effect of Antioxidant,” Stud. Conserv., vol. 36, no. 1, pp. 44–52, 1991, doi: 10.2307/1506451.
[2] Electronic Materials Handbook: Packaging. ASM International, 1989.
[3] M. Bilz and D. W. Grattan, The aging of parylene: difficulties with the arrhenius approach. 19960900, pp. 925–929.
[4] Hsi-wen Lo, Wen-Cheng Kuo, Yao-Joe Yang, and Yu-Chong Tai, “Recrystallized parylene as a mask for silicon chemical etching,” in 2008 3rd IEEE International Conference on Nano/Micro Engineered and Molecular Systems, Jan. 2008, pp. 881–884, doi: 10.1109/NEMS.2008.4484464.
Comments
Homepage 4/17/2020. 10:17:10 AM
... [Trackback] [...] Informations on that Topic: blog.paryleneconformalcoating.com/whats-the-difference-between-potting-and-conformal-coating/ [...]

londondrugscanada.bigcartel.comlondon-drugs 4/17/2020. 10:17:10 AM
cialis uk https://londondrugscanada.bigcartel.com/london-drugs This is nicely expressed. !