What Temperature Will My Parts See During the Parylene Coating Process?
Posted by Sean Horn
Friday, November 13, 2020 8:00
@ 8:00 AM
Parylene coating process involves 3 steps: sublimation, pyrolysis, and polymerization. At the first step, which takes place between 120°C – 175°C, the dimer parylene powder precursor is sublimized and pyrolysed forming the monomers (see Figure). Next, Pyrolysis takes place which is defined as the thermal decomposition of materials at elevated temperatures (above 500 °C) in an inert atmosphere and this reaction is irreversible. Finally, monomers get deposited onto the substrate and all the other surfaces available in the deposition chamber. Monomers form long chained polymers at this step.
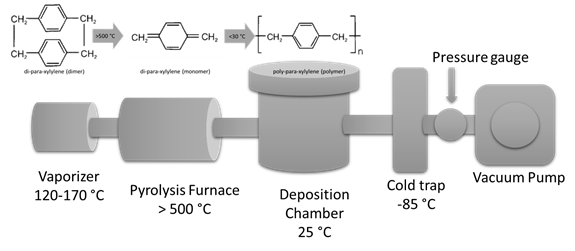
Polymerization route for poly-para-xylylene (Parylene N – C16H16)
What temperature is there in the deposition chamber?
Substrates to be coated are only found in the deposition chamber. Typically, the deposition chamber is kept at the ambient temperature so no heating is applied. Substrates found in this chamber are therefore not exposed to a temperature other than ambient temperature unless required by the process. Franz Selbmann et al showed that a slight increase of temperature during the process duration up to 40 °C was possible due to the hot monomer gas [1].
The steps that takes place from the time the monomer adsorbs onto the substrate to the polymerization is as follows [2]:
- adsorption of monomer onto thesubstrate
- surface migration and possibly bulk diffusion of monomer
- chemical reaction (propagation or initiation)
- Also desorption of monomer can occur any time after adsorption
Many models have been proposed to explain the chemical reaction (propagation and initiation) and the deposition rate as a function of monomer pressure and substrate temperature. Those models define the monomer concentration at the surface of the growing film using Flory-type adsorption, Langmuir-type adsorption, or Brunauer-Emmett-Tellertype (BET-type) adsorption. Fortin et al, developed a model based on kinetics and experimental work showing that the chemisorption model works well in predicting the deposition rate as a function of pressure and temperature for the steady state deposition of parylene-N [2]. They also claim that this model should apply to the other parylene-family polymers with the values of the fit parameters adjusted on the basis of the energetics of the Lennard-Jones potential for each monomer type.
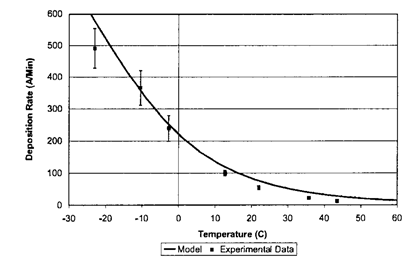
Rate vs Temperature at p=4 mTorr. Fit of the chemisorption model to the experimental data Image Ref: [2].
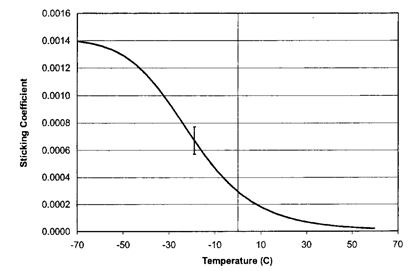
Sticking coefficient vs temperature from the chemisorption model. Image Ref: [2]
In the figure, the experimental data was fit to the model developed by the group. There is a good agreement between the two. At elevated temperatures, the deposition rate decreases quickly. This is explained by the lower reaction time of the monomer molecules with the reaction sites on the substrate.
A value of 0.001 indicates that 1 out of 1000 incident monomer molecules becomes part of the polymer and form the conformal coating. Sticking coefficient decreases as temperature increases as is also shown in the Deposition rate vs Temperature graph.
In conclusion, the typical temperature for the deposition chamber where the substrates lay during the parylene coating process is room temperature. This temperature may increase slightly up to 40 °C due to the incoming monomers. An increase in the deposition chamber temperature leads to a decrease in the deposition rate and sticking coefficient.
To learn more about parylene, download our whitepaper now
References
[1] F. Selbmann, M. Baum, M. Wiemer, and T. Gessner, “Deposition of Parylene C and characterization of its hermeticity for the encapsulation of MEMS and medical devices,” in 2016 IEEE 11th Annual International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Sendai, Japan, Apr. 2016, pp. 427–432, doi: 10.1109/NEMS.2016.7758283.
[2] J. B. Fortin and T.-M. Lu, “A Model for the Chemical Vapor Deposition of Poly( para -xylylene) (Parylene) Thin Films,” Chem. Mater., vol. 14, no. 5, pp. 1945–1949, May 2002, doi: 10.1021/cm010454a.
Comments
Homepage 4/17/2020. 10:17:10 AM
... [Trackback] [...] Informations on that Topic: blog.paryleneconformalcoating.com/whats-the-difference-between-potting-and-conformal-coating/ [...]

londondrugscanada.bigcartel.comlondon-drugs 4/17/2020. 10:17:10 AM
cialis uk https://londondrugscanada.bigcartel.com/london-drugs This is nicely expressed. !